New Insights on Urease Functioning

Preparation of a sample for cryo-electron microscopy analysis
A research group from the Department of Pharmacy and Biotechnology at the University of Bologna has uncovered a previously unknown element in the functioning of urease, a key enzyme in the medical and pharmaceutical field as well as in the agri-food sector. The results of the study—published in the International Journal of Biological Macromolecules—were obtained trough cryo-electron microscopy (cryo-EM), an imaging technique that freezes the sample to be observed at extremely low temperatures, allowing for a very high level of resolution, as in this case.
“Cryoelectronic microscopy opens up new perspectives on the mechanism of action of urease”, says Stefano Ciurli. “This new technique makes it possible to highlight fundamental aspects of enzyme catalysis that were inaccessible with X-ray crystallography, the technology previously employed to study these enzymes”.
Urease is a very widespread enzyme that requires the essential presence of two nickel atoms to play a key role in the biogeochemical cycle of nitrogen. Nickel is a highly toxic metal for humans, but in some biological systems it is indispensable and can therefore be considered an “essential poison”. Urease is the most efficient biological catalyst known and can accelerate the decomposition of urea by a hundred million billion times. Urea is a chemical compound (also found in blood and urine) widely used in agriculture as a fertiliser, but it releases large amounts of ammonia, thus producing air pollution.
In addition, urease is also found in plants, algae, fungi and is a virulence factor for many microorganisms, including several pathogens dangerous to human health that can develop antibiotic resistance. Knowing closely the working mechanism of this enzyme could therefore pave the way for the development of new drugs and molecules able to modulate its activity.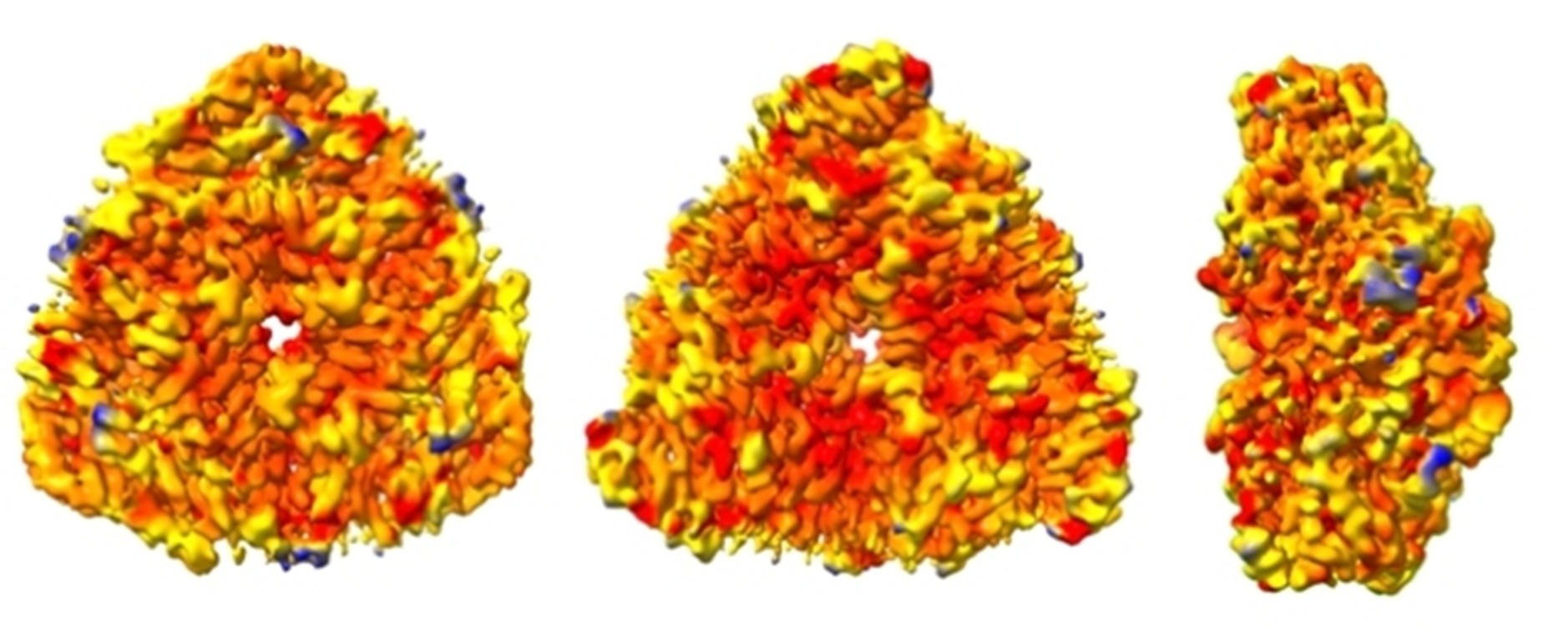
Representation of the surface of the structure of the urease enzyme (front, rear, and side view) derived from the bacterium Sporosarcina pasteurii, obtained using cryoelectronic microscopy. The different colouring of the representation describes regions with different mobility (red colour describes rigid regions, blue colour mobile regions)
Thanks to the use of cryoelectronic microscopy, researchers were able to "observe" the urease as it moves through space, identifying a previously unknown aspect of its action. Access to the place where the enzymatic reaction takes place is regulated by a dynamic movement of a portion of the protein that until now had been observed only in two conformations, open or closed. The new analysis has shown that there is also a third possible intermediate state between the two previously known.
“We have compared two urease structures found in a bacterium: one in its native form and the other inhibited by a molecule used in agriculture”, says Luca Mazzei, researcher from the Department of Pharmacy and Biotechnology and first author of the study. “In this way, we were able to detect the existence of a transient conformation between the open inactive state and the closed, catalytically active state”.
The discovery paves the way for new drugs and molecules that can inhibit and control the action of urease, with possible medical and therapeutic applications.
The study, published in International Journal of Biological macromolecules and entitled “Exploring the conformational space of the mobile flap in Sporosarcina pasteurii urease by cryo-electron microscopy”, was conducted with the participation of Stefano Ciurli and Luca Mazzei from the Department of Pharmacy and Biotechnology of the University of Bologna, together with Giancarlo Tria (Department of Chemistry of the University of Florence) and Michele Cianci (Department of Agricultural, Food and Environmental Sciences of the Università Politecnica delle Marche).





